Blog
Welcome to the section of Sabal Food Safety Consulting's web page dedicated to brainstorming discussions about food safety and related topics. Feel free to leave your comments or opinions about the topics under discussion. Should you enconter any difficulty using the Blog, send us an email to This email address is being protected from spambots. You need JavaScript enabled to view it. so we can investigate the problem.
Over here you'll see all publicly shared articles. Should you want to leave a comment on any article, click on the title and another window will open with the article and, at the bottom, there is a section where you can share your comments.
You could also register as a member and you'll receive notifications when new articles are published and, you'll have access to the articles developed only for registered members. Registration is free.
It is nice to have you here!
HACCP History Research

Validation and Verification, what do they mean?
 Sabal Food Safety Consulting's Blog / April 6th, 2014
Sabal Food Safety Consulting's Blog / April 6th, 2014System Effectiveness
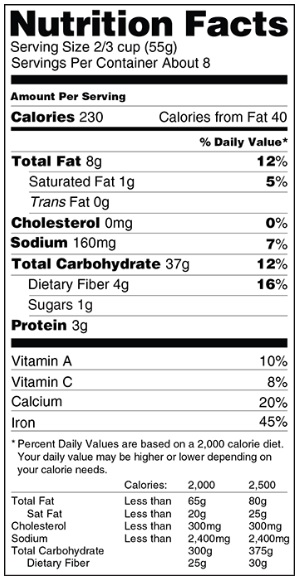
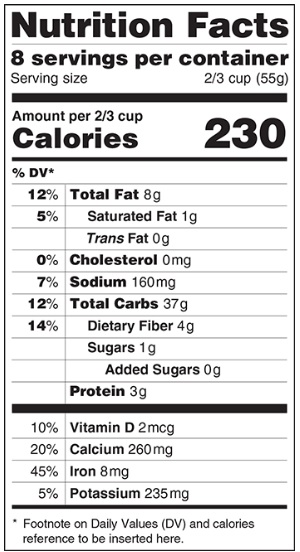
Nutrition Label - Proposed Changes
 Sabal Food Safety Consulting's Blog / March 1st, 2014
Sabal Food Safety Consulting's Blog / March 1st, 2014
Source: FDA's web site
Explanation of the proposed changes to the nutrition label in food products.
FDA wants to update the look and content of the Nutrition Facts Label to better help consumers make informed food choices and follow healthy dietary practices. The proposed changes include: a) a refreshed design, b) an updated "serving sizes" and "new package labeling requirements" and, c) the listing of "added sugars" and other changes based on nutrition science.
The FDA's web page states "A lot has changed in the American diet since the Nutrition Facts label was introduced in 1993 to provide important nutritional information on food packages. People are eating larger serving sizes. Rates of obesity, heart disease and stroke remain high. More is known about the relationship between nutrients and the risk of chronic diseases."
Summary of proposed changes:
- A greater emphasis—with larger and bolder type—on calories.
- Added sugars would be included on the label.
- Calories from fat would no longer be listed. Total, saturated and trans fat will still be required.
- The number of servings per package would also be more prominent. "Amount Per Serving" would now have the actual serving size listed, such as "Amount per cup".
- Updating serving size requirements. By law, serving sizes must be based on what people actually eat, not on what they "should" be eating.
- Update Daily Values for various nutrients and the %DV would be shifted to the left of the label.
- If present, the amounts of potassium and Vitamin D would be required on the label and, no longer require the labeling of Vitamins A and C.
The HACCP Team
What is the HACCP Team
Basically, anyone looking to implement a HACCP Program must first go through the five preliminary steps. For this discussion, we are going to talk about the HACCP Team.
The first step is to assemble a HACCP Team. The HACCP Team must be integrated by people that are knowledgeable of the process(es) performed and the product(s) manufactured. All members of the Team must be trained in the HACCP method, specially the HACCP Team Leader or HACCP Coordinator.
But why all members must understand the HACCP method?
When someone takes a training on HACCP, the basic information on how the HACCP method works and the definitions of hazards and risk are explained. The definition of a "Critical Control Point" (CCP), critical limit, what is a deviation?, corrective actions, verification and validation must be fully understood by the team members.
What happen when I'm auditing? I verify that the HACCP Coordinator/HACCP Team Leader has formal training and, that this training was examinable to determine basic understanding of the methodology. What is the expectative for the other team members? They must understand the HACCP method too! how can they cooperate in the development of the HACCP Program if they don't understand what is a hazard analysis, what is a hazard and which are its risks? All members of the HACCP Team must fully understand the basic concepts of the HACCP method!
Who else must have an understanding of the HACCP method? Monitors of CCPs and anyone responsible for verification of any area of the HACCP Program. What is your opinion about an employee that is a monitor of a CCP and does not know what is a deviation, does not know what is the critical limit or which are the parameters that are monitored and why?
Training on HACCP and verification of understanding of the training is of critical importance for the proper implementation of a HACCP Program.
What is your experience in assembling the HACCP Team?




